Numerous documented cases of hypoxia, hypercapnia, and hypoventilation have been associated with the use of pop-off valves, which create more risks than they solve. When used on patients with high airway resistance — a common condition during CPR — they can restrict necessary airflow, risking inadequate ventilation and compromising patient survival. In critical moments, these limitations may do more harm than good, underscoring the need for caution in their use.
As a recent Australian study stated: “we are concerned about the efficacy of pressure-limiting valves in emergency settings and the difficulties in identifying associated ventilatory problems” (1).
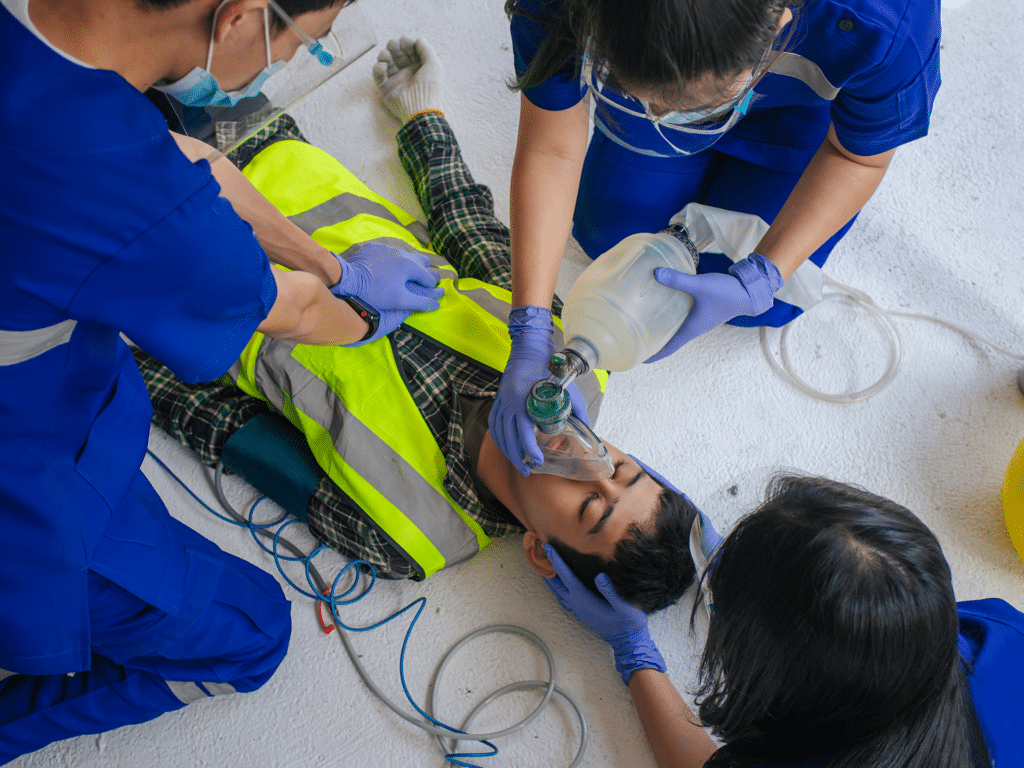
Three recent case reports (1-3) have investigated the consequences of using pressure limiting devices (pop-off valves) during manual ventilation on different types of patients. Eight cases were reported in these articles. Seven of them concerned adult patients, either experiencing out-of-hospital cardiac arrest (OHCA), overdose (OD), or respiratory failure. The last case involved a three-year-old girl who suffered from OHCA.
In all eight cases, patients were ventilated using manual resuscitators with a pop-off valve. Most of these patients presented with flat capnograph traces and oxygen desaturation despite the resuscitation efforts of the caregivers. The treated patients exhibited high respiratory resistance, stemming from compromised airways, respiratory pathologies, or muscular atrophy. These patients required high inspiratory pressure levels to overcome their respiratory resistance and deliver an adequate tidal volume. Pop-off valves typically limit the inspiratory pressure to below 40-60 cmH2O, depending on the model, and release excess pressure above this threshold.
Consequently, these eight patients did not receive an adequate tidal volume until caregivers noticed that the pop-off valve was leaking, which occurred up to 10 minutes after resuscitation efforts began. This resulted in hypoventilation and subsequent hypoxia for the patients.
These cases highlight the potential dangers of pressure limiting devices in manual ventilation, especially when used on patients with high respiratory resistance. They warn that such devices cannot be relied upon alone to guarantee the quality of manual ventilation, as there is a high risk of leading to hypoventilation and potential hypoxia.

Therefore, the only solution to guarantee safe and effective manual ventilation lies in the use of Ventilation Feedback Devices (VFDs).
EOlife is the only VFD able to calculate the tidal volume, the volume of gas currently reaching the lungs at each insufflation. It therefore appears as a unique chance to improve ventilation quality, and consequently, patient outcomes.
- Humar, M., Meadley, B., Groombridge, C., Cresswell, B., Anderson, D., & Nehme, Z. (2024). Bag-valve-mask resuscitators fitted with pressure-limiting valves-Safety feature or potential hazard?. Resuscitation plus, 20, 100789. https://doi.org/10.1016/j.resplu.2024.100789
- Rauschenbach, A., Pothireddy, S., Young, P., Reardon, R. F., & Driver, B. E. (2024). The Pitfalls of Using Pop-Off Valves in Adult Emergency Airway. The Journal of emergency medicine, 66(3), e361–e364. https://doi.org/10.1016/j.jemermed.2023.10.016
- Driver, B. E., Atkins, A. H., & Reardon, R. F. (2020). The Danger of Using Pop-Off Valves for Pediatric Emergency Airway Management. The Journal of emergency medicine, 59(4), 590–592. https://doi.org/10.1016/j.jemermed.2020.06.020
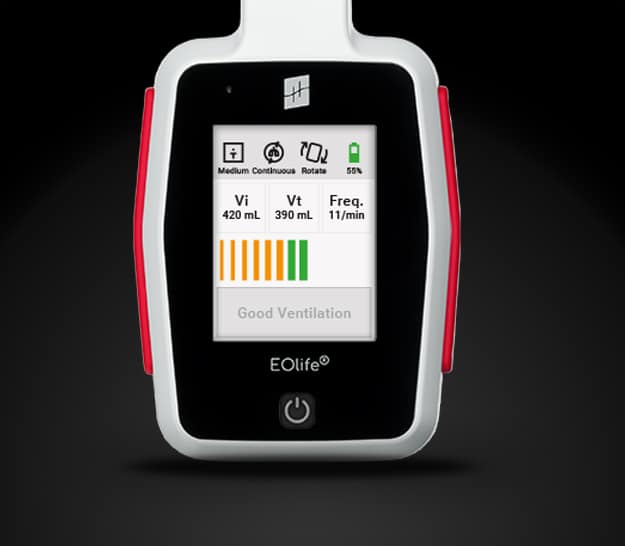
The ultimate medical device for high-performance manual ventilation.
Measure and adjust in real-time the quality of your manual ventilation. Medical device designed for the manual ventilation of adult patients in cardiopulmonary arrest.

The ultimate training tool for high-performance manual ventilation.
Record your training sessions. Analyze your ventilation cycles. Improve your manual ventilation practice.


