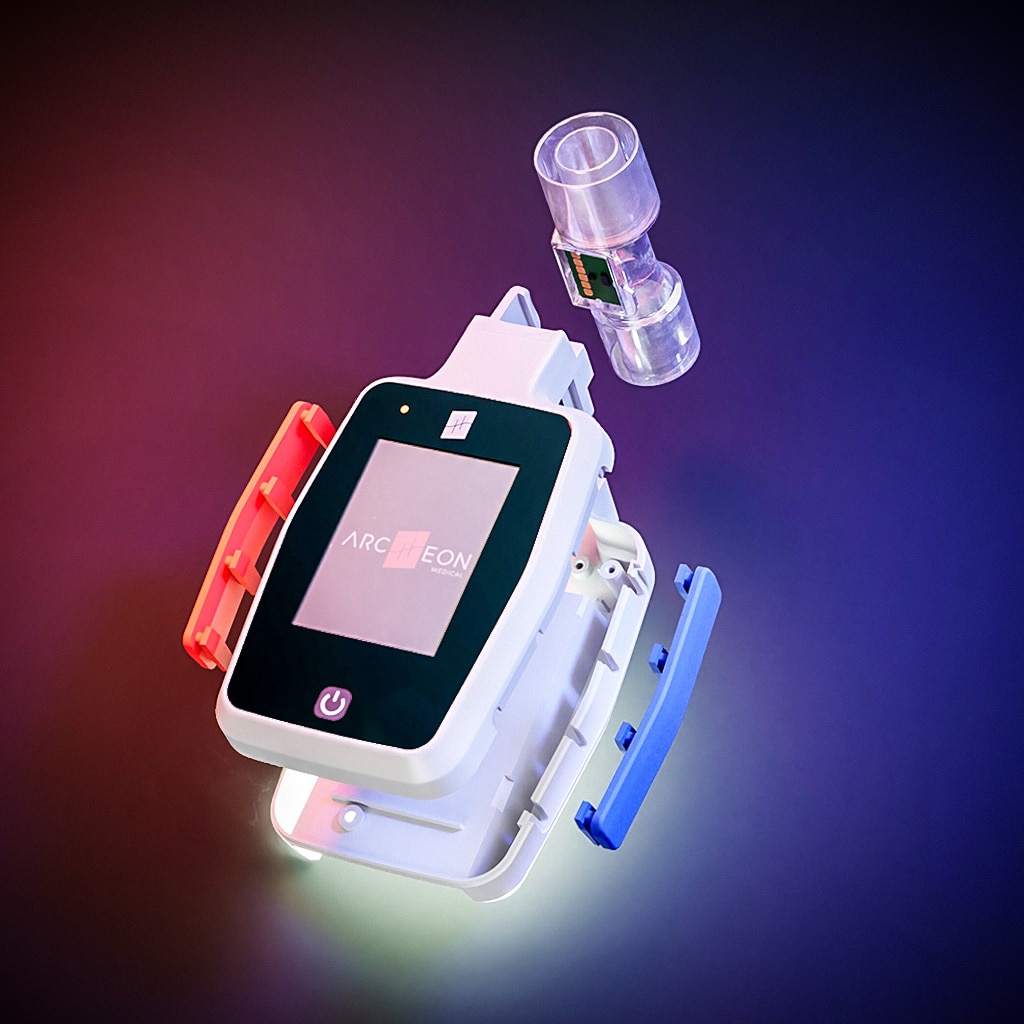
The only ventilation feedback device (VFD) to measure the volume of gas reaching the patient's lungs (TIDAL VOLUME)
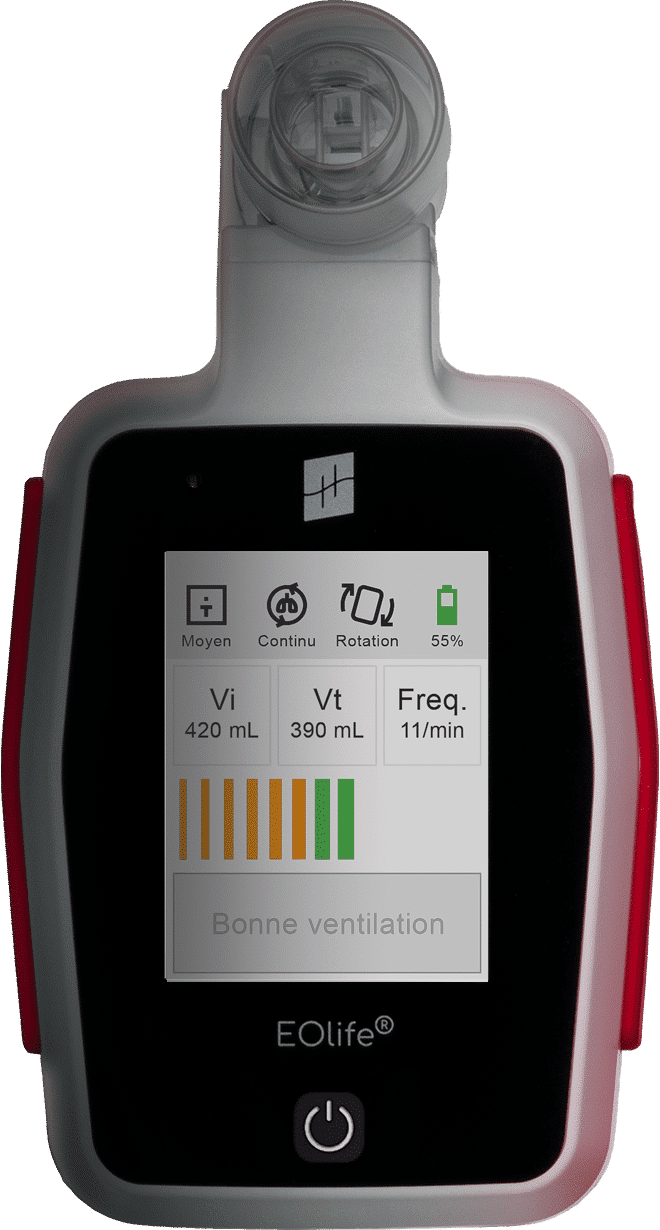
EOlife
improves
manual ventilation quality
by over 70% (I)

References
- Etude: Idris, A. H., Aramendi Ecenarro, E., Leroux, B., Jaureguibeitia, X., Yang, B. Y., Shaver, S., ... Wang, H. E. (2023). Bag-Valve-Mask Ventilation and Survival From Out-of-Hospital Cardiac Arrest: A Multicenter Study. Circulation, 148. DOI: 10.1161/CIRCULATIONAHA.123.065561.
Reference
Etude: Idris, A. H., Aramendi Ecenarro, E., Leroux, B., Jaureguibeitia, X., Yang, B. Y., Shaver, S., ... Wang, H. E. (2023). Bag-Valve-Mask Ventilation and Survival From Out-of-Hospital Cardiac Arrest: A Multicenter Study. Circulation, 148. DOI: 10.1161/CIRCULATIONAHA.123.065561.
Reference
Etude: Idris, A. H., Aramendi Ecenarro, E., Leroux, B., Jaureguibeitia, X., Yang, B. Y., Shaver, S., ... Wang, H. E. (2023). Bag-Valve-Mask Ventilation and Survival From Out-of-Hospital Cardiac Arrest: A

Since 2019, the Paris Fire Brigade has been involved in the development of EOlife through a multi-year partnership. Dr. Daniel Jost immediately believed in the project and the value of such technology for first responders: 'EOlife® addresses a significant gap in the management of cardiac arrest.

«We have decided to deploy the EOlife system on all ambulances from the SDIS, in the entire Doubs region. The EOlife provides tangible benefits to our firefighters and paramedics in their management of OHCA patients. EOlife is a valuable pre-hospital device that allows our 3,000 firefighters to improve the quality of their manual ventilation and to now provide high-performance ventilation. »

April 2023. Archeon has received U.S. Food and Drug Administration (FDA) clearance for its EOlife, the first smart device that measures the quality of manual ventilation. This approval marks a significant milestone for Archeon on its mission to promulgate the practice of high-performance ventilation.
extreme conditions
EOlife is designed for use in all types of conditions.


-4°F and 122°F maximum


run time
battery



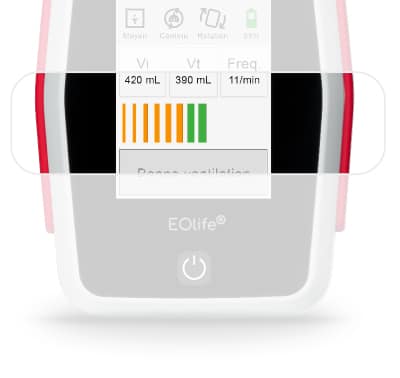
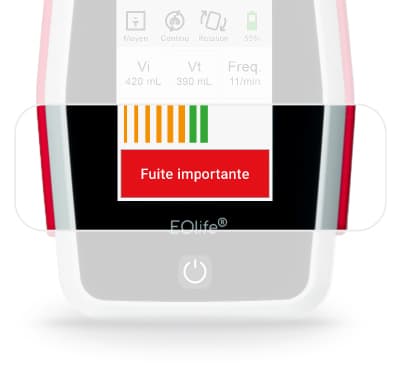


sensor
FlowSens is a biocompatible digital flow sensor. It is single use to eliminate contamination risks between patients.
sensor
Volume measurements are based on FlowSense® sensor measurements and are expressed in mL for the BTPS (body temperature and pressure, saturated).
The measurement accuracies of the values displayed on the screen are as follows:
- Vi (volume insufflated): ± 4.9% of the actual value measured under normal conditions of use
- Vt (tidal volume) without leakage: ± 5.5% of the actual value measured under normal conditions of use
- Freq (ventilation frequency): ± 1 cycle per minute
FlowSense® data:
- Flow range: ± 250 slm (standard litre per minute)
- Dead space: < 10 ml
Note: Certain types of ventilation bags can affect the measurement accuracy due to their design (non-laminar outgoing air flow). A slight measurement deviation may be observed but has no impact on compliance with regulatory requirements.
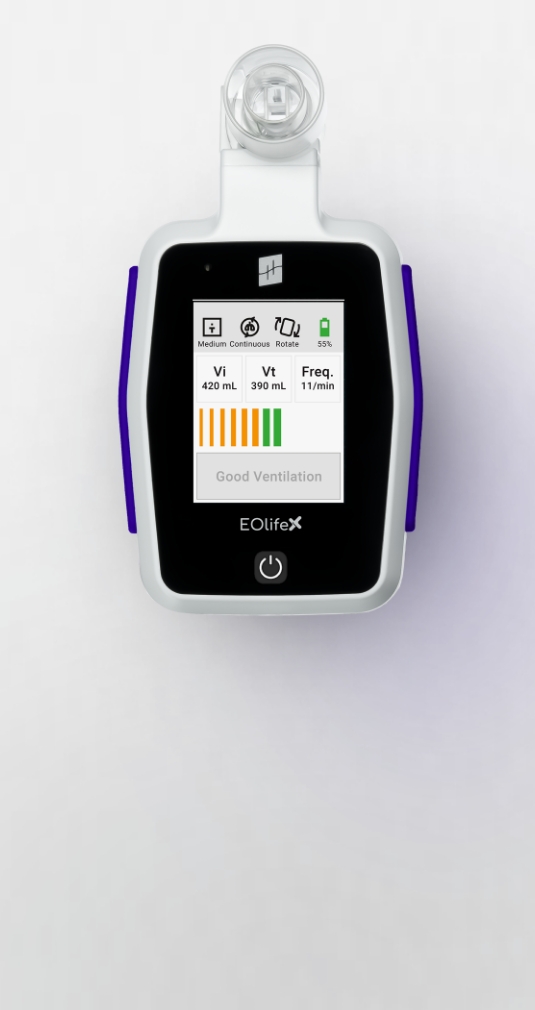
The ultimate training tool for high-performance manual ventilation.
Record your training sessions. Analyze your ventilation cycles. Improve your manual ventilation practice.